Table of contents:
- The drug development process in Canada
- What exactly is CNODES?
- How does CNODES decide what to research?
- Small-scale research versus large-scale queries
- Why not just pool all data together?
- Where does HQC fit into the CNODES picture?
In this post, we will cover what the Canadian Network for Observational Drug Effect Studies – or CNODES – does and how they inform decisions made by policy makers, health care providers, regulators, and patients on the safety and effectiveness of drugs.
Read on to learn more:
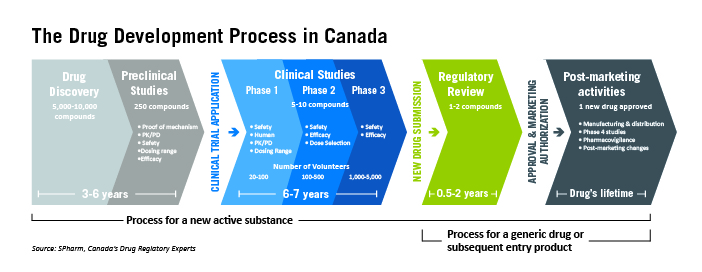
The drug development process in Canada
Before prescription or non-prescription drugs are made available to the Canadian public, they go through a rigorous review and approval process. This process – which involves experts reviewing the safety, effectiveness, and quality of the drug – takes an average of 12 years to complete.
Despite this process, an estimated 150,000 Canadians are hospitalized and 10,000 die each year from adverse drug reactions to prescription drugs. Adverse drug effects were also found to account for 12% of emergency department visits.
This is why drug safety research is so important.
While smaller-scale administrative health care databases have long been used to assess the risk and benefits of prescription and non-prescription drugs, a larger-scale approach is needed for urgent, less common, severe, or long-term adverse effects that can’t be detected by the clinical trials that are needed for initial drug approval.
This is where CNODES comes in.
What exactly is CNODES?
CNODES is a distributed network of Canadian researchers and data centres that work together to respond to research questions about the safety and effectiveness of drugs marketed in Canada.
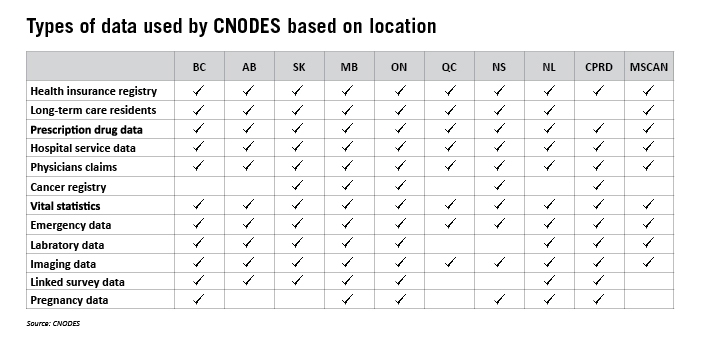
CNODES uses population databases from eight Canadian provinces and two international databases (UK Clinical Practice Research Datalink (CPRD) and US MarketScan), which all-told include more than 100 million patients.
With offices in British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, Nova Scotia, and Newfoundland and Labrador, these labs conduct research independently of each other, but study results inform a collective response to overarching research queries.
The different labs also collect different information, so every location might not be involved in every research study. Here’s what each location collects:
CNODES’ research studies have important implications and have been published in highly-ranked, peer-reviewed international journals.
How does CNODES decide what to research?
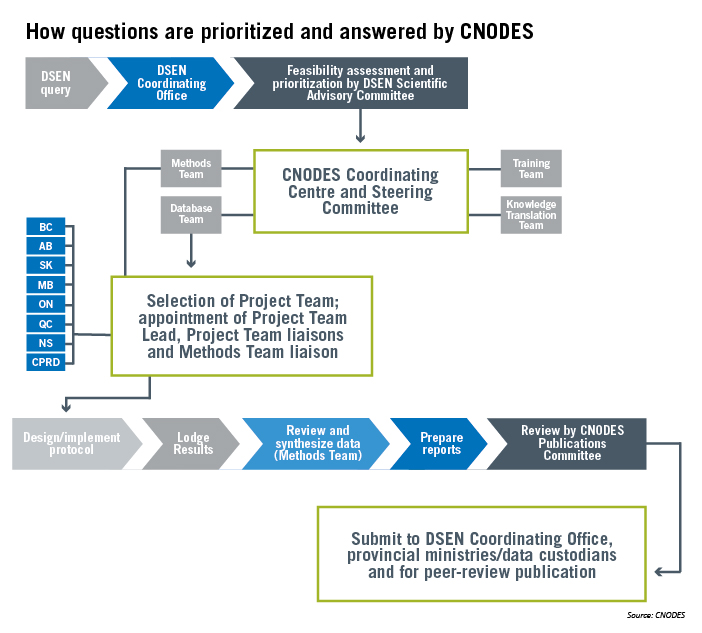
The research questions the CNODES network focus on come from Health Canada and are directed to an organization called the Drug Safety and Effectiveness Network (DSEN). DSEN assesses the research question before prioritizing it and sending it along to CNODES for consideration.
If CNODES finds the study feasible, a project team is selected from nominees with relevant expertise from each provincial data repository in the network, and processes are put in place to make sure researchers are using a consistent approach towards designing their studies and analyzing the results.
Small-scale research versus large-scale queries
Most studies on drug safety and effectiveness are conducted outside of the CNODES network, where research is done on a smaller scale with smaller groups. These administrative health care databases have long been used to evaluate adverse drug effects, but responses to drug safety signals have been slow and uncoordinated.
For example, safety concerns came up in 2000 about the adverse cardiovascular effects of rofecoxib, a drug used to treat arthritis pain that was prescribed to more than 80 million people worldwide. Research was conducted by separate teams of researchers using databases in Ontario, Quebec, and Saskatchewan. It took between three and nine years for each group to respond to the first report on safety concerns, and investigators used different approaches in designing their studies and analyzing their results. Because they were working independently of each other, there were discrepancies in the results and estimated risk to individuals wasn’t accurate.
That’s not to say there isn’t a place for this smaller-scale research, but it does mean there is a strong need for an urgent response larger-scale queries through a collective network like CNODES.
Why not just pool all data together?
Legal and privacy concerns make it impractical to pool the data from the different provinces. This distributed network instead allows data to stay in the provinces of origin and be analyzed locally.
The upside is that local regulations concerning data access and approval processes for research are respected, and local know-how is supported, but the downside is that there can be delays in accessing data or obtaining approvals for individual studies.
Where does HQC fit into the CNODES picture?
The Health Quality Council (HQC) is the Saskatchewan site of the national CNODES network. HQC participates in CNODES as part of its role in evaluating and monitoring prescription drug usage, safety and effectiveness.
HQC’s CNODES team has been involved on projects regarding:
- The use of systemic oral fluoroquinolones in Canada
- Direct oral anticoagulants for the treatment of venous thromboembolic events
- The utilization and comparative effectiveness of rheumatoid arthritis medications
- The utilization and adverse outcomes of ondansetron and fluconazole therapy during pregnancy
- The utilization, effectiveness, and safety of SGLT2 inhibitors among patients with type 2 diabetes mellitus
Related materials:
CNODES DESN Brochure (winter 2019)